Protein Aggregation And Neurodegenerative Disease
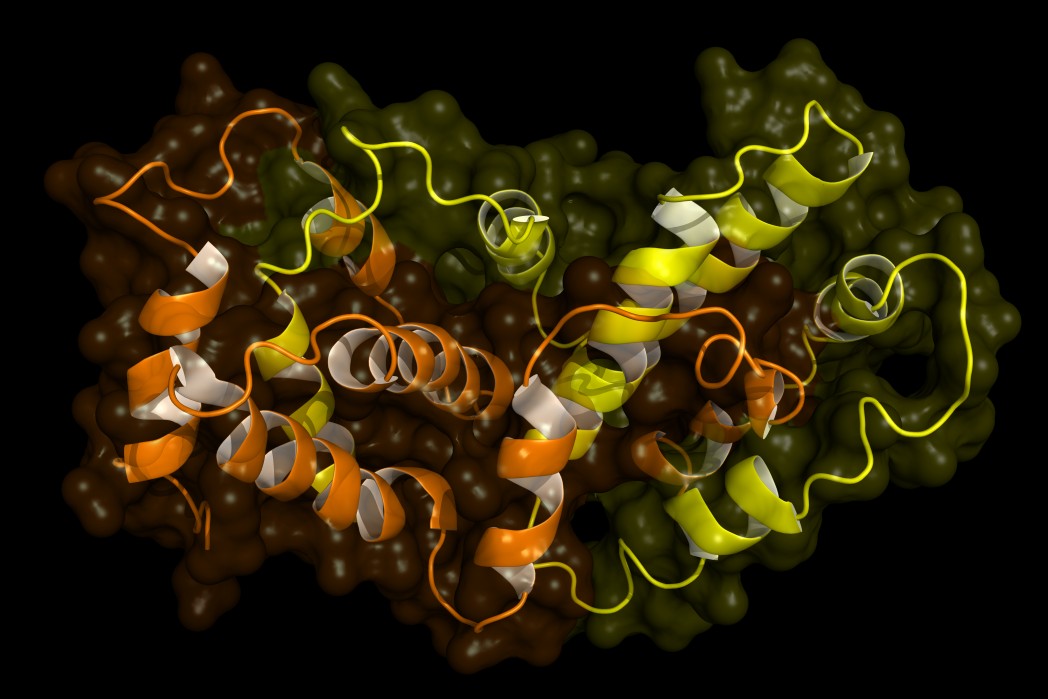
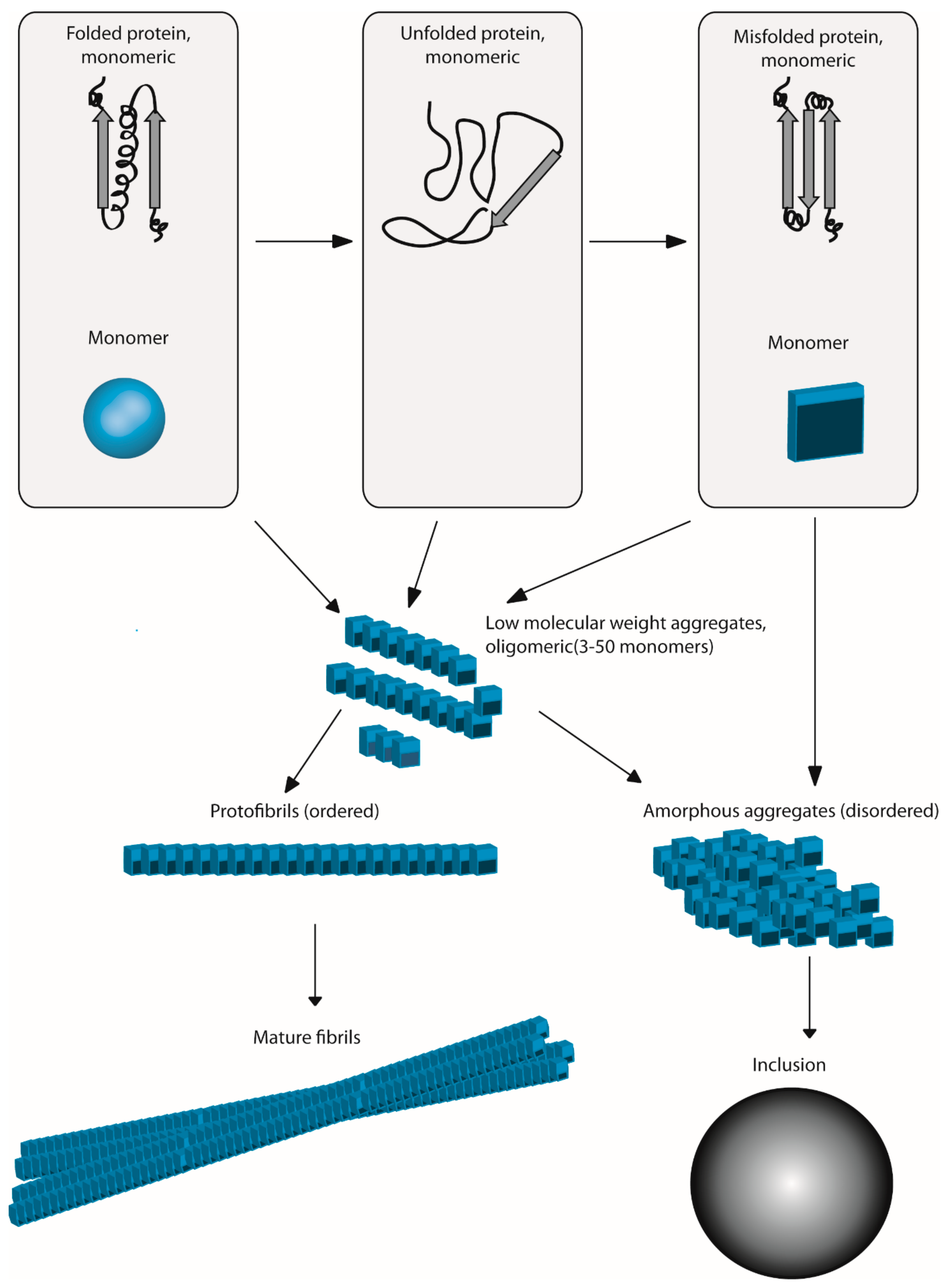
Protein aggregation and neurodegenerative disease. They are characterized by severe neuronal losses in several disease-specific brain regions associated with deposits of aggregated proteins. Protein misfolding leads to protein aggregation and accumulation of these aggregates is implicated as the main reason of neurodegenerative diseases. Therefore it has been suggested that protein aggregation is pathogenic.
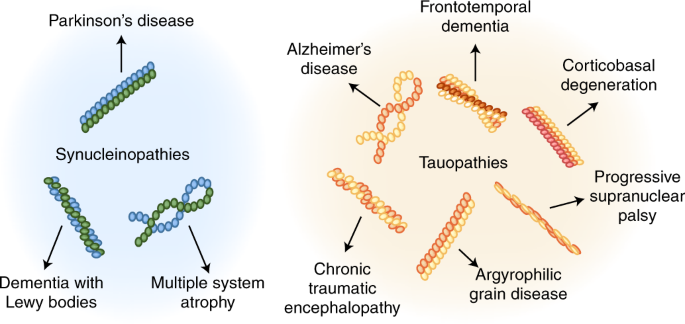
The protein aggregates are observable in a number of neurodegenerative diseases as extracellular deposits in Alzheimers AD and prion disease or as intracellular aggregates in Parkinsons PD and Huntingtons disease HD. Read more related scholarly scientific articles and abstracts. Alzheimers and Parkinsons diseases are the most prevalent neurodegenerative diseases that generate important health-related direct and indirect socio-economic costs.
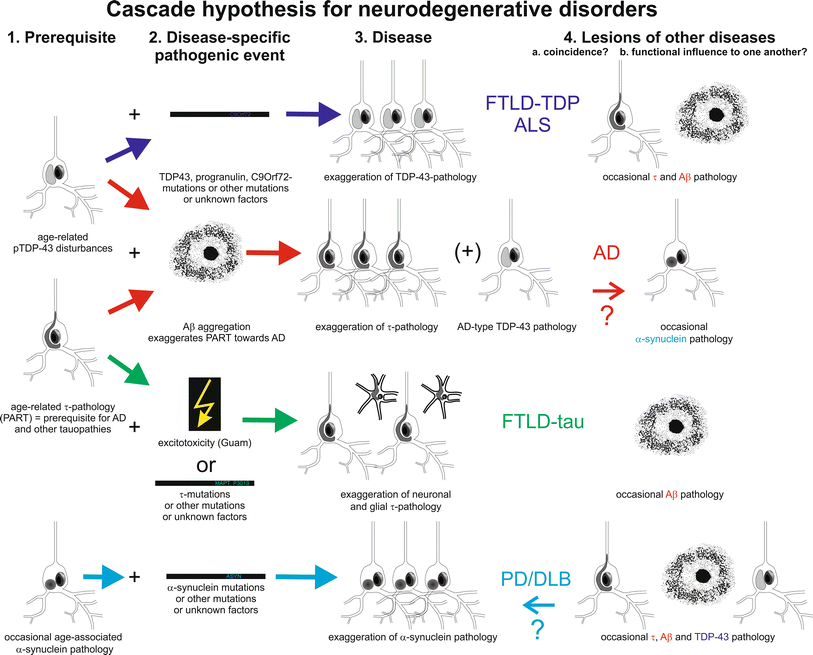
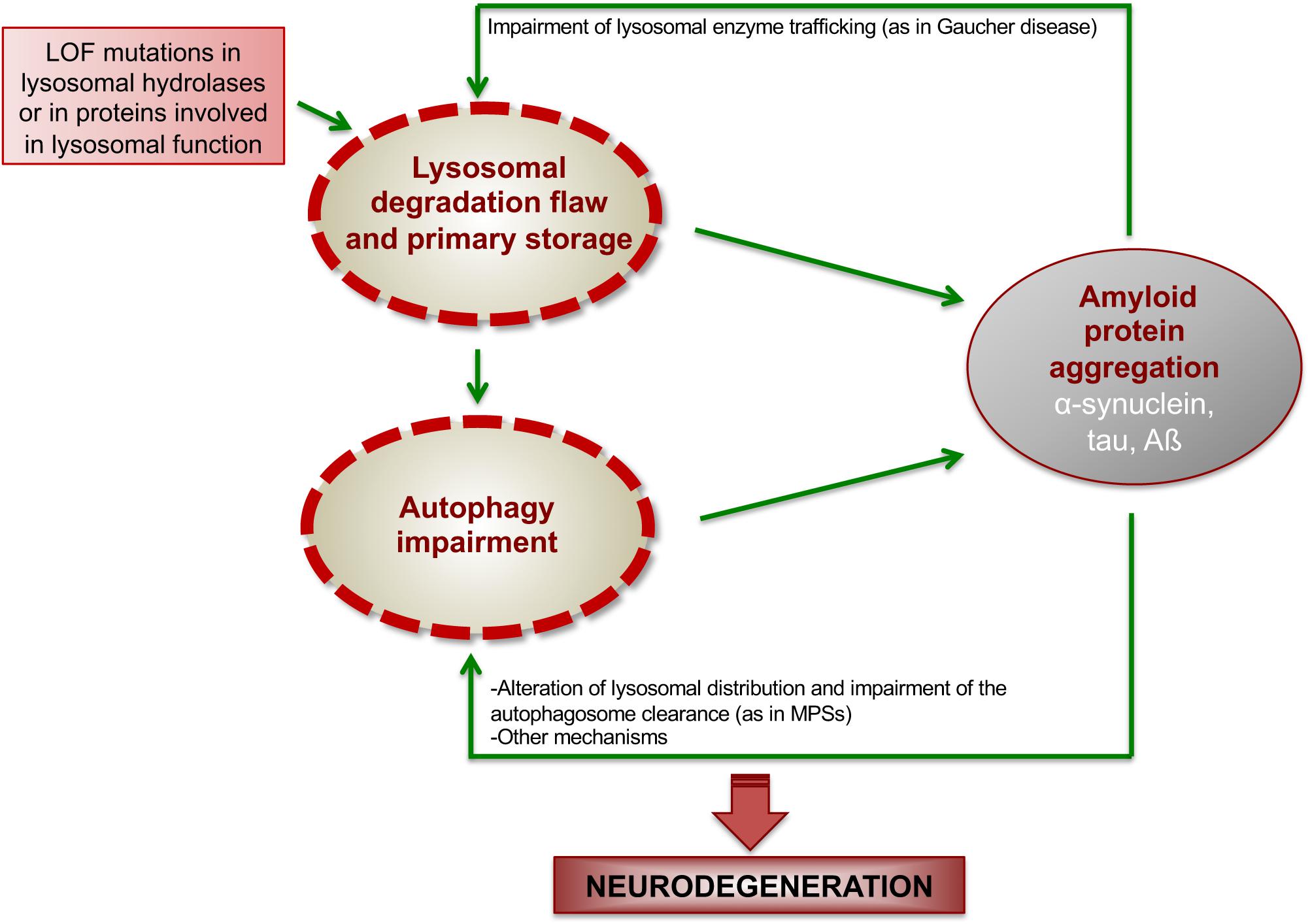
Intraneuronal protein aggregation as a trigger for inflammation and neurodegeneration in the aging brain Age is by far the greatest risk factor for Alzheimers disease AD yet few AD drug candidates have been generated that target pathways specifically associated with the aging process itself. Most common among them is neurodegenerative disorder in which the protein abnormally aggregate and deposit in specific regions of the brain and hampers its function Table1. The neuroendocrine protein 7B2 suppresses the aggregation of neurodegenerative disease-related proteins.
In an unfavorable cellular environment protein may get misfolded resulting in its aggregation. Indeed abnormal protein aggregation characterizes many if not all neurodegenerative disorders not just AD and Parkinsons disease but also CreutzfeldtJakob disease motor neuron diseases the large group of polyglutamine disorders including Huntingtons disease 1 as well as diseases of peripheral tissue like familial amyloid polyneuropathy FAP. The onset or progression of disease that occurs due to formation of protein aggregates is called as protein aggregation diseases.
Neurodegenerative diseases such as Alzheimers disease AD Parkinsons disease PD Huntingtons disease HD amyotrophic lateral sclerosis ALS and prion diseases. Protein aggregation underlies several neurodegenerative diseases such as Alzheimers Huntingtons chorea or Parkinsons. Protein aggregation and neurodegenerative disease Christopher A Ross Michelle A Poirier Neurodegenerative diseases such as Alzheimers disease AD Parkinsons disease PD Huntingtons disease HD amyotrophic lateral sclerosis ALS and prion diseases are increasingly being realized to have common cellular and molecular mechanisms.
The manifestation of a disease is usually dependent on the specific brain region that the neurotoxicity affects. Neurodegenerative diseases are identified as protein misfolding diseases proteopathies and protein conformational disorders. Neurodegenerative diseases typically involve deposits of inclusion bodies that contain abnormal aggregated proteins.
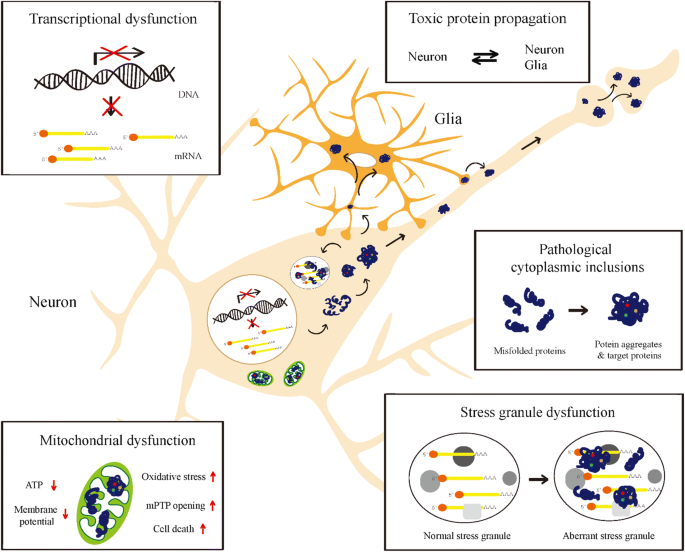
Misfolded proteins often aggregate and accumulate to trigger neurotoxicity through cellular stress pathways and consequently cause neurodegenerative diseases. Protein misfolding leads to protein aggregation and accumulation of these aggregates is im.
Read more related scholarly scientific articles and abstracts.
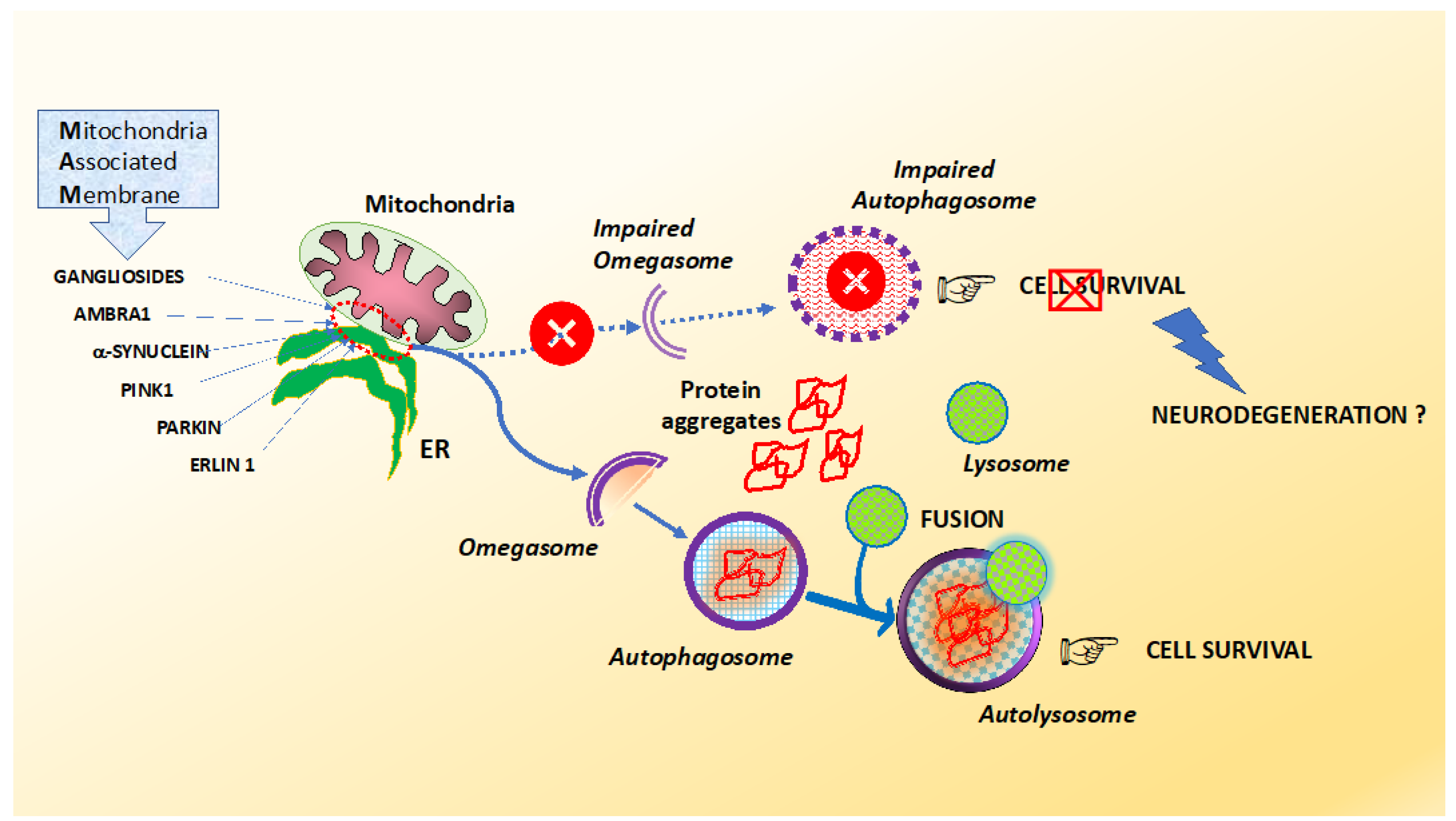
Misfolded proteins often aggregate and accumulate to trigger neurotoxicity through cellular stress pathways and consequently cause neurodegenerative diseases. Alzheimers and Parkinsons diseases are the most prevalent neurodegenerative diseases that generate important health-related direct and indirect socio-economic costs. Neurodegenerative diseases typically involve deposits of inclusion bodies that contain abnormal aggregated proteins. The protein aggregates are observable in a number of neurodegenerative diseases as extracellular deposits in Alzheimers AD and prion disease or as intracellular aggregates in Parkinsons PD and Huntingtons disease HD. The neuroendocrine protein 7B2 suppresses the aggregation of neurodegenerative disease-related proteins. Protein aggregation and neurodegenerative disease Christopher A Ross Michelle A Poirier Neurodegenerative diseases such as Alzheimers disease AD Parkinsons disease PD Huntingtons disease HD amyotrophic lateral sclerosis ALS and prion diseases are increasingly being realized to have common cellular and molecular mechanisms. In brain some native proteins prion tau β-amyloid α-synuclein and huntington undergo conformational changes via genetic and environmental factors. Misfolded proteins often aggregate and accumulate to trigger neurotoxicity through cellular stress pathways and consequently cause neurodegenerative diseases. Most common among them is neurodegenerative disorder in which the protein abnormally aggregate and deposit in specific regions of the brain and hampers its function Table1.
Most common among them is neurodegenerative disorder in which the protein abnormally aggregate and deposit in specific regions of the brain and hampers its function Table1. Neurodegenerative diseases are identified as protein misfolding diseases proteopathies and protein conformational disorders. Intraneuronal protein aggregation as a trigger for inflammation and neurodegeneration in the aging brain Age is by far the greatest risk factor for Alzheimers disease AD yet few AD drug candidates have been generated that target pathways specifically associated with the aging process itself. Misfolded proteins often aggregate and accumulate to trigger neurotoxicity through cellular stress pathways and consequently cause neurodegenerative diseases. Indeed abnormal protein aggregation characterizes many if not all neurodegenerative disorders not just AD and Parkinsons disease but also CreutzfeldtJakob disease motor neuron diseases the large group of polyglutamine disorders including Huntingtons disease 1 as well as diseases of peripheral tissue like familial amyloid polyneuropathy FAP. Most common among them is neurodegenerative disorder in which the protein abnormally aggregate and deposit in specific regions of the brain and hampers its function Table1. In case of the α-synuclein this protein is natively folded and normally water soluble in the cell.

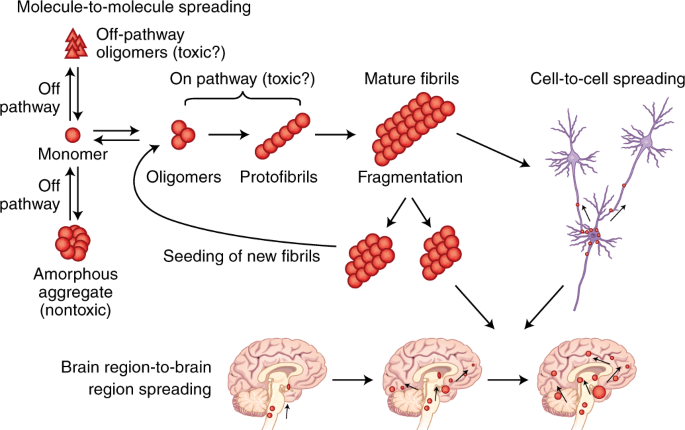



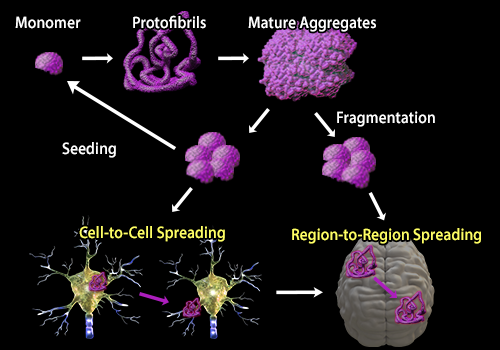

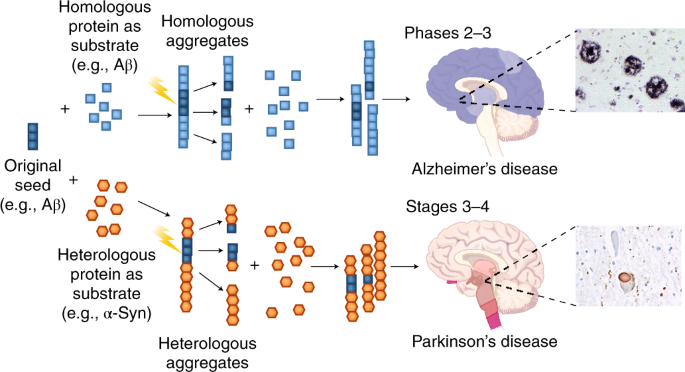
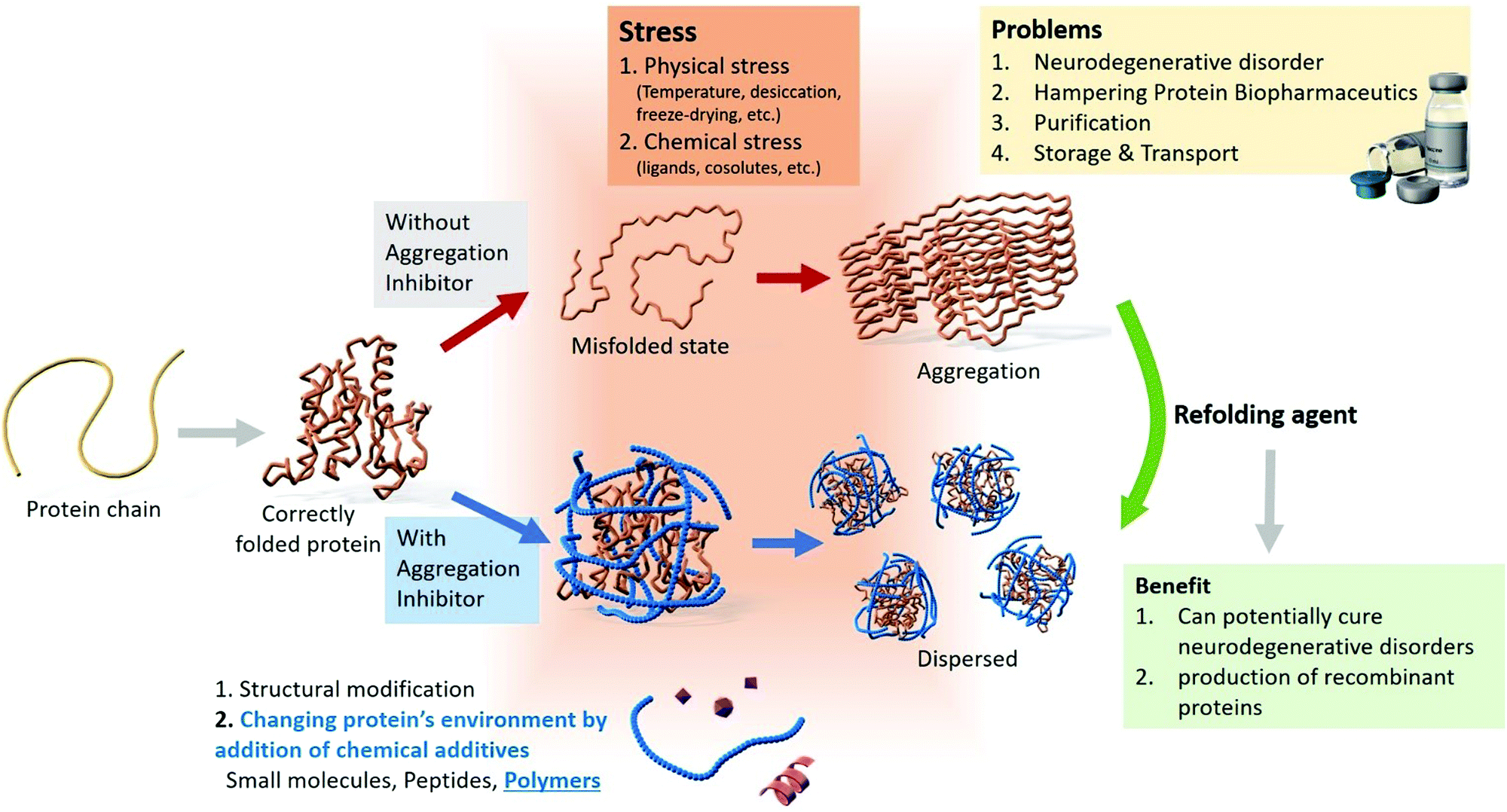























Post a Comment for "Protein Aggregation And Neurodegenerative Disease"