Sprix Nasal Spray Discontinued
Sprix nasal spray discontinued. We have used only the nasal spray. It is formulated as a liquid 10 mg ketamine per 01 mL. Sprix nasal spray has not been discontinued.
Contact your doctor if you experience headache. SPRIX should be discontinued immediately in patients with skin reactions. It is used by mouth by nose by injection into a vein or muscle and as eye drops.
The burning was far worse than the migraine and I could not keep using this without lidocaine nasal gel or spray. Throw the bottle away 24 hours after your first use even if there is still medicine left inside. Virtus discontinued ketorolac in March 201912 FDA imposed an import ban in mid-2013 on several Wockhardt products including ketorolac13 Sprix.
At the time I bought Sprix I did. Or swelling of ankles feet or hands. Are great to work with and the scripts are delivered in a refrigerated package.
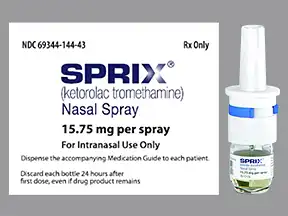
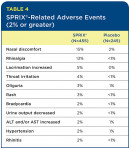
Common side effects include sleepiness dizziness abdominal pain swelling and nausea. Sprix Ketorolac tromethamine NA 1575 mg One 1575 mg spray in each nostril every 6 to 8 hours OR For special populations. 54 56 NSAIDs can cause serious dermatologic adverse reactions such as exfoliative dermatitis.
Can reach them at 1-866-823-5184. Do not use any ketorolac products including this nasal spray tablets or injection for more than a total of 5 days. During pregnancy use of SPRIX beyond 30 weeks gestation can cause premature closure of the ductus arteriosus resulting.
Store unopened nasal spray. The decrease in headache tends to be short-lived.
Epidermal necrolysis which can be fatal.
Contact your doctor if you experience headache. Contact your doctor if you experience headache. It is used by mouth by nose by injection into a vein or muscle and as eye drops. Each bottle of this medicine contains 8 sprays for use within a 24-hour period. It works like a regular mail order pharmacy. One 1575 mg spray in only one nostril every 6 to 8 hours for up to 5 days This document contains proprietary and confidential information that should not be released to. Virtus discontinued ketorolac in March 201912 FDA imposed an import ban in mid-2013 on several Wockhardt products including ketorolac13 Sprix. Common side effects include sleepiness dizziness abdominal pain swelling and nausea. SPRIX should be discontinued immediately in.
It is formulated as a liquid 10 mg ketamine per 01 mL. Hope this helps. SPRIX should be used with caution in patients with cardiac decompensation or similar conditions. Do not use any ketorolac products including this nasal spray tablets or injection for more than a total of 5 days. Recommended duration of treatment is less than six days. Persons with disabilities having problems accessing the PDF files below may call 301 796-3634 for assistance. Do not use Sprix for longer than 5 days unless your doctor has told you to.




































Post a Comment for "Sprix Nasal Spray Discontinued"